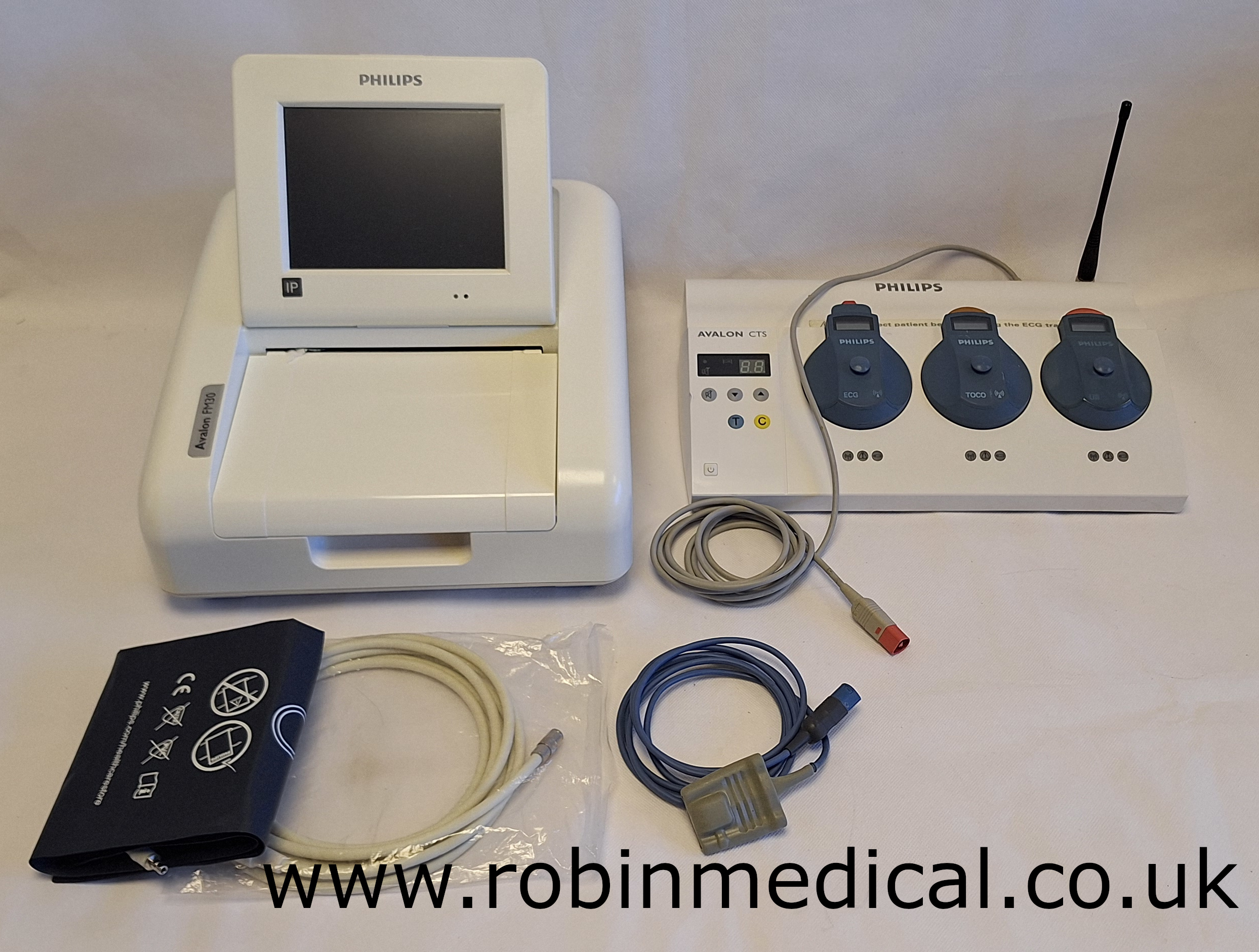
Philips Avalon FM 30 Foetal Monitor with CTS Fetal Transducer Base Station, including ECG, TOCO & Ultrasound Transducer, SpO2 and NIBP with printing functionality
2 in stock
£2,000.00
2 in stock
Philips Avalon FM 30 Foetal Monitor with CTS Foetal Transducer Base Station, including ECG, TOCO & Ultrasound Transducer, SpO2 and NIBP with printing functionality
Avalon FM foetal and maternal monitors are the first and only ones from Philips to provide automated coincidence detection (cross-channel verification) utilising Smart Pulse, recording foetal and maternal heart rates independently.
- Model: Philips Avalon FM 30
- Supplied with:
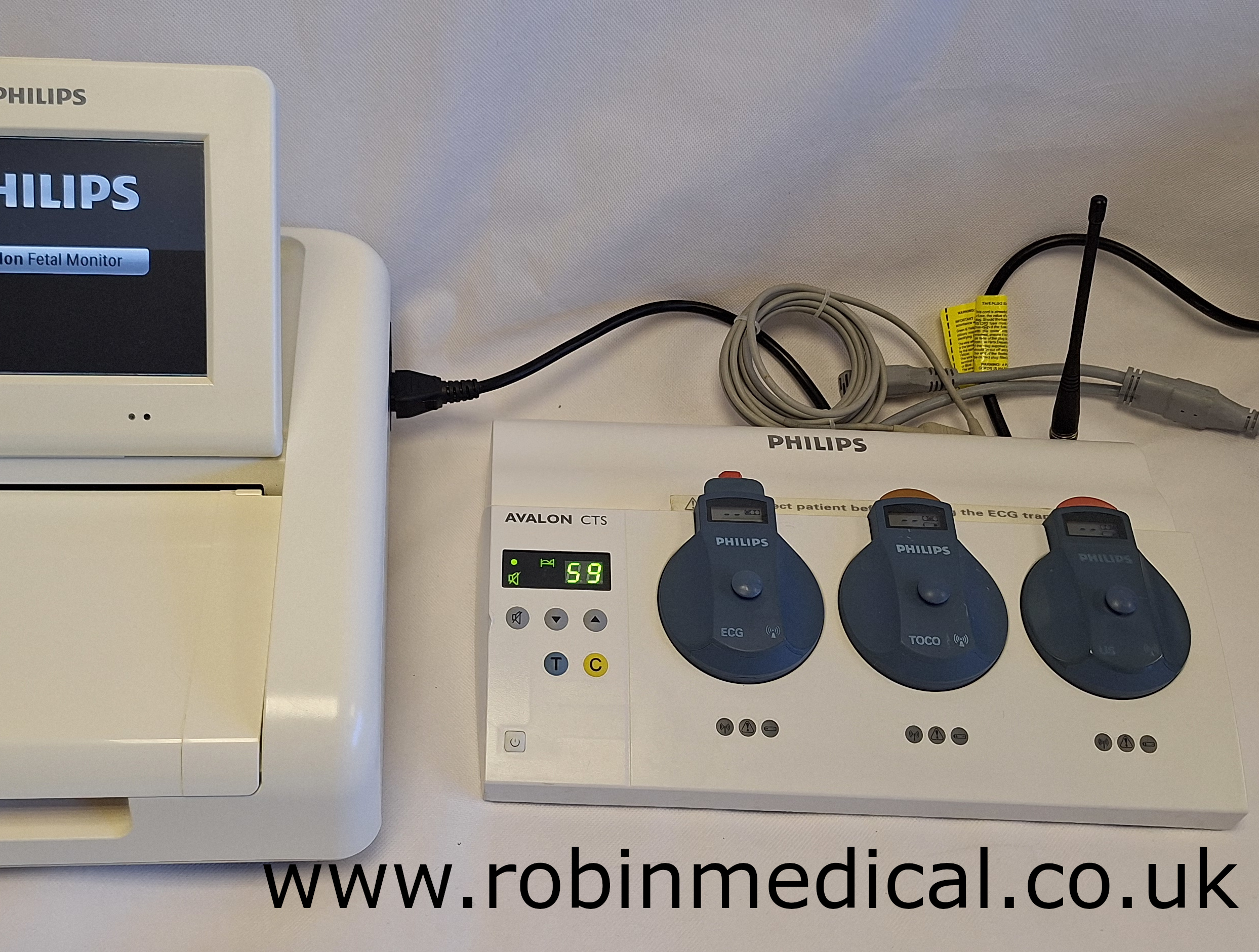
- This comes with the CTS foetal transducer base station for enabling wireless monitoring (M2720A);
- Philips Toco Transducer (M2725A);
- Philips Wireless Ultrasound Transducer (M2726A);
- Philips Wireless ECG Transducer (M2727A);
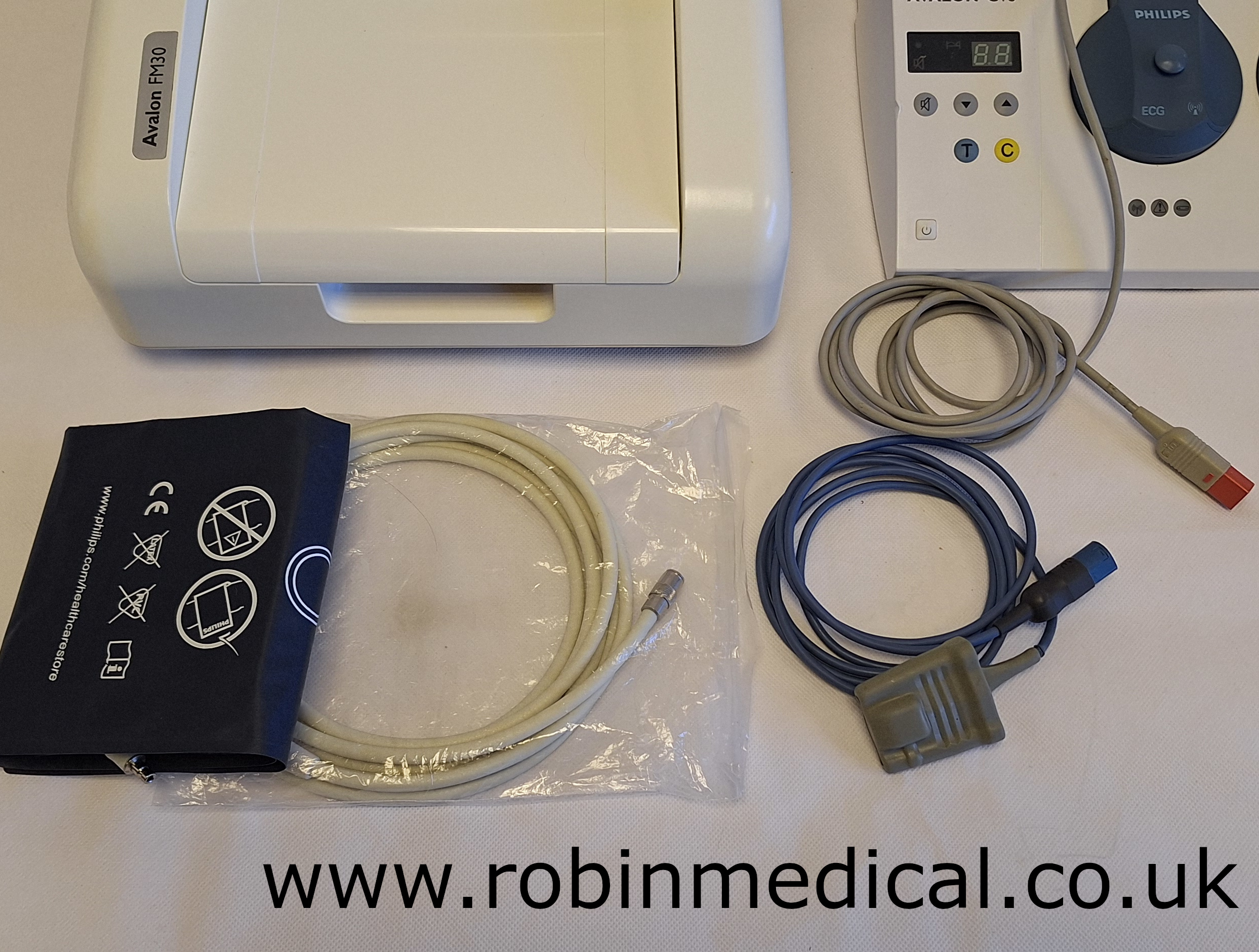
- NIBP hose and cuff;
- Philips SpO2 sensor cable;
Immediately available for dispatch, patient ready and this product is offered with a 3 month warranty period.
The Philips Avalon FM30 combined with the CTS Fetal Transducer Base Station delivers a fully integrated maternal‑fetal monitoring solution. Designed for labor and delivery as well as antepartum and postpartum care, this system supports simultaneous tracking of fetal heart rate (via ultrasound), uterine activity (TOCO), maternal ECG, SpO₂, and non‑invasive blood pressure. The CTS Base Station provides seamless transducer management and automatic signal routing, while the integrated thermal printer ensures real‑time strip chart documentation.
Technical Specifications
| Specification | Details |
|---|---|
| Model | Avalon FM30 + CTS Fetal Transducer Base Station |
| Manufacturer | Philips |
| System Compatibility | Avalon series monitors |
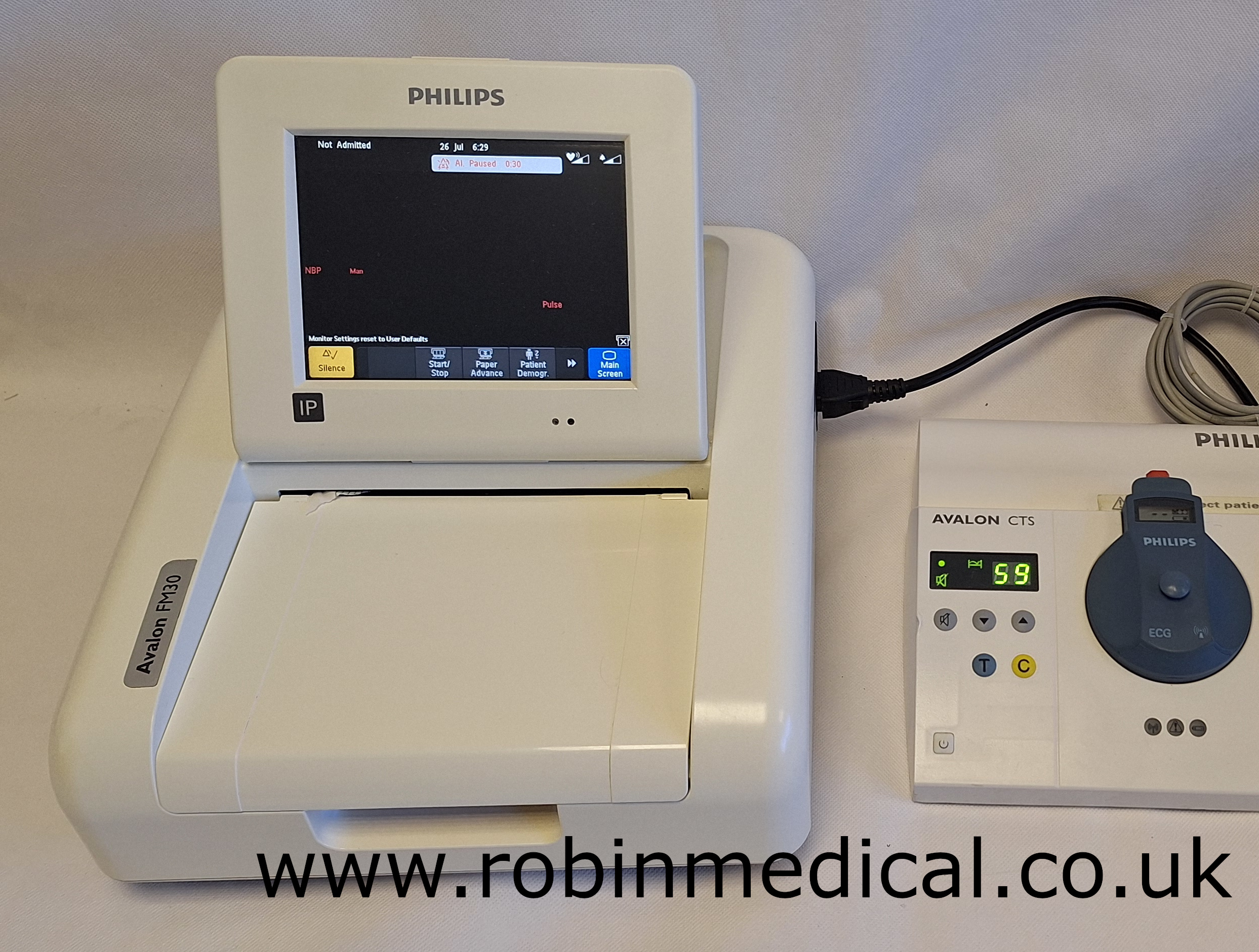
| Display | 6.5″ Color TFT touchscreen |
| Fetal Monitoring Channels | Up to 3 FHR channels (Ultrasound) |
| Uterine Activity (TOCO) | External strain‑gauge transducer |
| Maternal ECG | 3‑lead ECG |
| SpO₂ | Integrated pulse oximetry |
| NIBP | Non‑invasive blood pressure (auto/manual modes) |
| CTS Base Station | Automatic transducer detection and signal routing |
| Data Display | Real‑time numeric and waveform (FHR, TOCO, ECG) |
| Printing | Built‑in thermal printer (150 mm strip width) |
| Alarms | Visual and audible for all parameters |
| Connectivity | USB, Serial, Optional LAN/Wi‑Fi |
| Data Storage | Internal memory + external USB export |
| Power Supply | AC 100–240 V, 50/60 Hz; internal rechargeable battery backup |
| Battery Backup | Up to 2 hours (monitor only) |
| Mounting Options | Tabletop, Wall, Cart mount |
| Dimensions (W × H × D) | 35 × 15 × 30 cm |
| Weight | Approx. 6.0 kg |
| Certifications | CE, FDA 510(k), ISO 13485 |
Key Features
-
Integrated CTS Base Station
Automatically recognizes and routes signals from each fetal and uterine transducer, reducing setup time and cable clutter. -
Comprehensive Maternal‑Fetal Monitoring
Simultaneously tracks three fetal heart rates, uterine contractions, maternal ECG, SpO₂, and NIBP on a single platform. -
Intuitive Touchscreen Interface
6.5″ color display with easy‑to‑navigate menus and waveform review. -
Real‑Time Printing
Continuous thermal strip chart provides immediate hard‑copy records of all monitored parameters. -
Smart Alarm Management
Configurable visual and audible alarms for early warning of fetal or maternal distress. -
Flexible Data Connectivity
USB, serial, and network options support seamless integration with EMR and central monitoring systems. -
Portable & Battery‑Backed
Internal battery ensures uninterrupted monitoring during power interruptions or patient transport. -
Clinical‑Grade Reliability
Built to withstand demanding clinical environments with rigorous quality and safety certifications.